Before the Test:
Rest for at least eight hours.
Eat a healthy breakfast.
Hydration.
Review Notes
Better sleep and healthier breakfast meals wake you up to be open-minded during the test.
If you are open-minded, you will socre a better score.
During the Test:
Most commona B and C
Multiple Choice: Process of Elimination. Eliminate irelevant answers.
If you are not finished when time is called over, guess the rest of the answers, if there are no penalties for missing questions. Chances are you will get at least one of them right.
Read the question again and again. Look for tricks like "does not".
Some Chemistry questions are not established with reason so choose the best answer.
Good luck on your test!
Jose Guerrero Chemistry P1
Wednesday, April 25, 2012
Wednesday, April 11, 2012
Molarity, Molarity and Percent Concentration
I'll show YOU how it's done!
Example: What is the molarity of a solution prepared by dissolving 60.0 g of sodium hydroxide in enough water to make a total of 550 mL of solution?
Method:
Example: What is the molarity of a solution prepared by dissolving 60.0 g of sodium hydroxide in enough water to make a total of 550 mL of solution?
Method:
- Calculate the number of moles of solute present. mol NaOH=60.0
g NaOHx1 mol NaOH40.0g NaOHmol NaOH = 1.5 mol NaOH
- Calculate the number of liters of solution present. L soln=550
mLx1 L=0.55 L soln1000mL - Divide the number of moles of solute by the number of liters of solution.
M = 1.5 mol NaOH= 2.7 M NaOH 0.55 L soln
Wednesday, March 21, 2012
LE CHATELIER'S PRINCIPLE
Le Chatelier's principle describes what happens to a system when something momentarily takes it away from equilibrium. This section focuses on three ways in which we can change the conditions of a chemical reaction at equilibrium:
(1) changing the concentration of one of the components
(2) changing the pressure
(3) changing the temperature at which the reaction is run
To show this, consider the steam reforming reaction used in previous experiments.
C (s) + H2O (g) 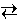 CO (g) + H2 (g)
CO (g) + H2 (g)
In this experiment the volume of the system can be changed and the corresponding changes in equilibrium amounts of carbon, water, carbon monoxide, and hydrogen can be observed.

http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch16/graphics/16_11fig.gif
(1) changing the concentration of one of the components
(2) changing the pressure
(3) changing the temperature at which the reaction is run
To show this, consider the steam reforming reaction used in previous experiments.
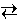 CO (g) + H2 (g)
CO (g) + H2 (g)In this experiment the volume of the system can be changed and the corresponding changes in equilibrium amounts of carbon, water, carbon monoxide, and hydrogen can be observed.
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch16/graphics/16_11fig.gif
Wednesday, March 7, 2012
Gas Stoichiometry Problems
Graham's Law
Example 4:
Hydrogen sulfide reacts with sulfur dioxide to give H2O and S, H2S + SO2 = H2O + S(solid), unbalanced. If 6.0 L of H2S gas at 750 torr produced 3.2 g of sulfur, calculate the temperature in C. Solution
Balanced reaction:
Atomic mass: H, 1.0; O, 16.0; S, 32.0. R = 0.08205 L atm /(K mol) is OK but watch units used for pressure. source: http://www.science.uwaterloo.ca/~cchieh/cact/c120/gastoichiometry.html
Example 4:
Balanced reaction:
2 H2S + SO2 = 2 H2O + 3 S(solid),
2 mol 3*32 = 96 g
2 mol H2S
3.2 g S ---------- = 0.067 mol H2S;
96 g S
P = 750/760 = 0.987 atm
PV 0.987 atm * 6 L
T = --- = --------------------------------
n R 0.067 mol * 0.08205 atm L /(mol K)
= 1085 K
= 812°C
Discussion:Atomic mass: H, 1.0; O, 16.0; S, 32.0. R = 0.08205 L atm /(K mol) is OK but watch units used for pressure.
Graham's Law of Effusion
Graham's Law
Mathematically, this can be represented as:
Graham's Law shows the relationship between the molar or molecular mass of a gas and the rate at which it will effuse. Effusion is the process of gas molecules escaping through tiny holes in their container. Diffusion can also be considered with Graham's Law, such as perfuming diffusing through a room.
Let us first consider why gases effuse. Containers can have small holes or pores in them. Although these openings are microscopic, they are larger than the gas molecules. Randomly, the gas molecules move around the inside of the container until they impact something. This can be another molecule or the side of the container. A gas can also, instead of hitting the side of the container, pass through one of those openings by chance. This is effusion: a random movement of a as molecule through the container's wall. A common example of this is a balloon filled with helium: first it is buoyant and floats in the air, but in a few days it hangs toward the ground or floats a few inches above the ground (if at all). The Helium has escaped through the small holes in the balloon.
With Graham's Law, you can find the effusion rates for two gases or the molecular mass of a gas. This ratio of effusion rates follows the pattern that the gas with the lesser molecular mass has a greater rate of effusion.
Calculations using Graham's Law
Let's compare the rate off effusion of two common gases, Nitrogen and Oxygen. N2, Nitrogen, has a molecular mass of 28.0 g. O2, Oxygen, has a molecular mass of 32.0 g. Therefore, to find the ratio, the equation would be:
Let's try to find a molecular mass. Let's use gas A and B. A is 0.68 times as fast as B. The mass of B is 17 g. What is the mass of A?
First, we set up the equation. Plugging in the values to our formula, we get:
source=http://library.thinkquest.org/12596/graham.html
Wednesday, February 15, 2012
Wednesday, February 1, 2012
Chemistry Notes
1. Get yourself an unbalanced equation. Here's where you use your knowledge of formulas to help you out. If you know what the formula of sodium hydroxide, sulfuric acid, sodium sulfate, and water are, you'd be able to write the following unbalanced equation:

2. Draw boxes around all the chemical formulas. This is the step that people frequently don't do because they feel that it's a stupid thing to do. Those people are morons. Ignore them. You're drawing those boxes so that you'll be sure not to mess around with the formulas to balance the equation. While they all suffer in the pits of academic hell, you'll be laughing from the honor roll. Here's what the equation looks like:

3. Make an element inventory. In this inventory, your job is to figure out how many atoms of each element you have on the left and right sides of the equation. Now, if you look at the equation, you should be able to see that on the left side of the equation there is one sodium atom, five oxygen atoms (one from the sodium hydroxide, four from the sulfuric acid), three hydrogen atoms (one from the sodium hydroxide, two from the sulfuric acid), and one sulfur atom. On the right side of the equation, there are two atoms of sodium, one atom of sulfur, five atoms of oxygen (four from the sodium sulfate and one from the water), and two atoms of hydrogen. Thus, your element inventory should look like this:

4. Write numbers in front of each of the boxes until the inventory for each element is the same both before and after the reaction. Now, what happens when we put a number in front of a formula? Basically, anything in that box is multiplied by that number, because we're saying that we have that many of that kind of molecule. So, looking at the inventory, what should we do?
Well, we can see that on the left side of the inventory, there is one atom of sodium and on the right there are two. The solution: Stick a "2" in front of the sodium hydroxide on the left side of the equation so that the numbers of sodium atoms are the same on both sides of the equation. When we do this, the new atom inventory should look like this: (I'll let you figure out how this is done)

Now what? Well, looking at the new inventory, we can see that we now have two sodium atoms on both the left and the right sides, but the others still don't match up. What to do?
You can see from the inventory that on the right side of the equation, there are two hydrogen atoms and on the left there are four. Using your amazing powers of mathematics (and hopefully not needing to use a calculator), you can see that two multiplied by the number two becomes four. That's what you need to do. How? Put a "2" in front of the water on the right side of the equation to make the hydrogens balance out. Now that this is done, you should make a new inventory that looks something like this:

Since both sides of the inventory match, the equation is now balanced! All other equations will balance in exactly the same way, though it might take a few more steps in some cases.

Well, we can see that on the left side of the inventory, there is one atom of sodium and on the right there are two. The solution: Stick a "2" in front of the sodium hydroxide on the left side of the equation so that the numbers of sodium atoms are the same on both sides of the equation. When we do this, the new atom inventory should look like this: (I'll let you figure out how this is done)

You can see from the inventory that on the right side of the equation, there are two hydrogen atoms and on the left there are four. Using your amazing powers of mathematics (and hopefully not needing to use a calculator), you can see that two multiplied by the number two becomes four. That's what you need to do. How? Put a "2" in front of the water on the right side of the equation to make the hydrogens balance out. Now that this is done, you should make a new inventory that looks something like this:

Subscribe to:
Posts (Atom)
